Buy ARA-290 Peptide
Buy ARA-290, a nonerythropoietic peptide derived from erythropoietin, designed to reduce inflammation and support nerve repair without causing serious side effects.
Shop our Full Range of ARA-290 Peptides
-
Sale!

ARA-290 Pre-Mixed Pen 2mg
£10.91 – £37.91Price range: £10.91 through £37.91 This product has multiple variants. The options may be chosen on the product page -
Sale!

ARA290 Peptide Vial
£8.33 – £17.31Price range: £8.33 through £17.31 This product has multiple variants. The options may be chosen on the product page -
Sale!

ARA-290 Nasal Spray
£16.33 – £27.66Price range: £16.33 through £27.66 This product has multiple variants. The options may be chosen on the product page
What Is ARA-290?
ARA-290 is a small peptide derived from erythropoietin, a protein that is important for red blood cell production. Unlike erythropoietin, ARA-290 is designed to offer therapeutic benefits without affecting red blood cell levels. It primarily works by targeting specific receptors, such as the innate repair receptor (IRR). It helps promote tissue repair and reduce inflammation.
Studies suggest that the peptide may have potential in treating conditions like autoimmune diseases, chronic pain, and diabetic neuropathy. Its ability to reduce nerve inflammation and support healing makes it a promising candidate for further medical research.
ARA-290 Mechanism Of Action
It works by targeting and activating the innate repair receptor (IRR). The IRR receptor complex is formed by the beta common receptor and the erythropoietin receptor found on the surface of certain cells.
Once activated, this receptor triggers protective pathways in the body. Studies show it aids in reducing inflammation and supporting the repair of damaged tissues. By blocking pro-inflammatory signals, it helps prevent further tissue damage. It also encourages cell survival and regeneration.
ARA-290 exclusively focuses on its anti-inflammatory and regenerative properties. Unlike traditional erythropoietin, which stimulates red blood cell production and may cause unwanted side effects. This targeted mechanism makes it a safer and more effective option for long-term use in treating nerve-related conditions and chronic pain.
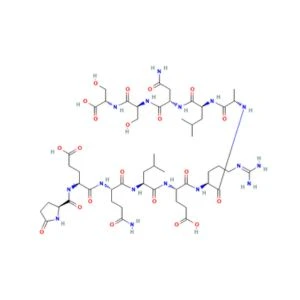
Structure of ARA-290
Sequence: H-Pyr-Glu-Gln-Leu-Glu-Arg-Ala-Leu-Asn-Ser-Ser-OH
Molecular Formula: C51H84N16O21
Molecular Weight: 1257.3 g/mol
PubChem CID: 91810664
What Are The Research Benefits of ARA-290
Relief for Neuropathic Symptoms: Research shows that the 11 amino acid erythropoietin derivitive may help reduce neuropathic symptoms in people with type 2 diabetes and sarcoidosis. It can be given through IV (intravenous) or subcutaneous administrationor (injections), making it a flexible treatment option [1].
Metabolic Control: Besides neuropathic symptom relief, it also exhibits promising effects on metabolic control in subjects with type 2 diabetes. This dual-action nature may make it an attractive therapeutic option for managing complex, co-morbid conditions [2].
Increase in Corneal Nerve Fiber Density: A study demonstrated that it can increase corneal nerve fiber density in patients with sarcoidosis-associated small nerve fiber loss [3]. This suggests that it has regenerative properties, possibly due to its action on the innate repair receptor.
Chronic Neuropathic Pain Treatment: It acts at the innate repair receptor, a heteromer of the erythropoietin receptor and β-common receptor. This mechanism of action has been associated with the alleviation of chronic neuropathic pain in sarcoidosis patients [4].
Promising SPECT Tracer for Cardiac Ischemia: It has been evaluated for its potential as a Single Photon Emission Computed Tomography (SPECT) tracer for targeting cardiac ischemic regions. The results suggest potential diagnostic benefits [5].
Treatment of Painful Diabetic Neuropathy: Clinical studies have shown the safety and efficacy of the peptide in treating painful diabetic neuropathy [6]. Further larger clinical trials are needed for more conclusive results.
Potential Neuroprotective Benefits: Apart from its analgesic properties, it also appears to have neuroprotective benefits, making it a potential treatment for post-stroke depression [6].
Attenuating Apoptosis and Inflammation: It has been studied for its role in attenuating apoptosis and inflammation in critical limb ischemia, suggesting its potential use in vascular diseases [7] [8].
Protection against Cytokine-Induced Apoptosis: Research has indicated that it can protect rat islets from cytokine-induced apoptosis [8], suggesting possible applications in autoimmune conditions.
Reducing Tissue Damage: Studies imply that it may reduce tissue damage by reducing inflammation [9].
Immune System Enhancement: it shows potential in immunomodulation by targeting the TPR pathway, possibly reducing inflammatory molecules like IL-6 and alleviating immuno-compromising conditions. Research also suggests it may influence adaptive immunity by altering antigen presentation in dendritic cells, potentially improving outcomes in transplantation models [10].
Buy ARA-290 for laboratory research use online today!
Buy ARA-290 Peptide Vial
Buy ARA-290 peptide vial 2 mg , a lyophilized powder for laboratory purposes. As a synthetic erythropoietin analogue, it activates the innate repair receptor without impacting red blood cell production. Available from Direct Peptides, it’s a reliable tool for studying inflammation and tissue repair therapies.
Buy ARA-290 Peptide Nasal Spray
Buy ARA-290 nasal spray , available in 15 ml and 30 ml sizes, offering flexibility for both short-term and long-term studies. The 15 ml size is ideal for trial use, while the 30 ml option is cost-effective for long term research studies. Both sizes deliver the same high-quality formulation in an easy-to-use nasal spray.
Frequently Asked Questions (FAQs) about ARA-290
What is ARA-290?
It is a synthetic peptide derived from erythropoietin, designed to harness its therapeutic benefits without the erythropoietic effects. It has been investigated for its potential in alleviating inflammation, reducing pain, and providing neuroprotective effects.
What conditions is ARA-290 being studied for?
It is being researched as a potential treatment for conditions such as neuropathic pain, critical limb ischemia, autoimmune diseases, and post-stroke depression. Its role in reducing tissue damage and inflammation has been a key focus.
How does ARA-290 work?
It appears to act through the innate repair receptor (IRR), a target linked to tissue protection and repair. By engaging this receptor, it may help reduce inflammation, apoptosis, and pain.
Is ARA-290 approved for medical use?
At present, it is still undergoing clinical research and has not yet been widely approved for medical treatment by the United States Food & Drug Administration (FDA). Its potential applications are promising but require further validation in FDA regulated clinical trials.
Are there any known side effects of ARA-290?
Preliminary research suggests that it is generally well tolerated, though more studies are needed to fully understand its safety profile and any potential long-term effects.
How is ARA-290 administered?
It is typically administered via subcutaneous injection in clinical trials. However, its dosing and administration specifics will depend on future approvals and medical guidelines.
What makes ARA-290 unique?
Unlike traditional erythropoietin therapies, itselectively targets the IRR without stimulating red blood cell production, making it a safer option for conditions where erythropoiesis is not desired.
What is erythropoiesis?
Erythropoiesis is the process by which red blood cells (erythrocytes) are produced in the body. This process primarily occurs in the bone marrow and is regulated by the hormone erythropoietin, which is released by the kidneys in response to low oxygen levels. Red blood cells play a crucial role in transporting oxygen to tissues and removing carbon dioxide, making erythropoiesis essential for maintaining overall physiological balance and proper organ function.
Buy ARA-290 2 mg Pre-mixed pen from Direct Peptides. Pre-Mixed Cartridge Kits include a premixed cartridge, pen, carry case, and needle tips. Single Mixed Cartridges come in options of 1, 2, or 3 cartridges with needle tips but no kit. Save 10% when purchasing 3 cartridges.
Summary of Research Applications
- Investigated for its anti-inflammatory properties in neurological disorders.
- Explored as a treatment for peripheral neuropathic pain conditions.
- Studied for promoting nerve repair and regeneration in various injury models.
- Evaluated in managing diabetic autonomic neuropathy.
- Assessed for its efficacy in addressing spared nerve injury.
- Considered a potential therapy for reducing neuroinflammation without adverse effects.
- Used in experimental studies to understand its benefits in nonerythropoietic peptides.
ARA-290 Peptide Quality Assured
At Direct Peptides, we provide ARA-290 peptides with 99% purity. Our careful synthesis process ensures each batch meets strict quality and effectiveness standards.
Our high-quality product supports innovative research into treatments for nerve-related conditions and chronic pain.
When you buy ARA-290 from us, you can count on a reliable compound designed to deliver consistent results for your research.
References For Further Reading
[1] Michael Brines, Ann N Dunne, Monique van Velzen, Paolo L Proto, et al (2015) ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes – Molecular Medicine, 2015 Mar 13; Volume 20 (Issue 1), Pages 658-66.
[2] Lara Heij, Marieke Niesters, Maarten Swartjes, Elske Hoitsma , et al (2012) Safety and Efficacy of ARA 290 in Sarcoidosis Patients with Symptoms of Small Fiber Neuropathy: A Randomized, Double-Blind Pilot Study – Molecular Medicine, 2012 Nov 15, Volume 18 (Issue 1), Pages 1430–1436.
[3] Albert Dahan, Ann Dunne, Maarten Swartjes, Paolo L Proto, Lara Heij, et al (2013) ARA 290 Improves Symptoms in Patients with Sarcoidosis-Associated Small Nerve Fiber Loss and Increases Corneal Nerve Fiber Density – Molecular Medicine, 2013 Oct 8, Volume 19 (Issue 1), Pages 334–345.
[4] Marieke Niesters, Maarten Swartjes, Lara Heij, Michael Brines, Anthony Cerami, et al (2012) The erythropoietin analog ARA 290 for treatment of sarcoidosis-induced chronic neuropathic pain – Expert Opinion on Orphan Drugs, 17 Dec 2012, Volume 1 (Issue 1), Pages 77-87.
[5] Naser Mohtavinejad, Maliheh Hajiramezanali, Mehdi Akhlaghi, Ahmad Bitarafan-Rajabi, and Nazila Gholipour (2021) Synthesis and evaluation of 99mTc-DOTA-ARA-290 as potential SPECT tracer for targeting cardiac ischemic region – Irananian Journal of Basic Medical Sciences, 2021 Nov, Volume 24 (Issue 11), Pages 1488–1499.
[6] Michael Brines, Ann N Dunne, Monique van Velzen, Paolo L Proto, Claes-Goran Ostenson, et al (2015) ARA 290, a Nonerythropoietic Peptide Engineered from Erythropoietin, Improves Metabolic Control and Neuropathic Symptoms in Patients with Type 2 Diabetes – Molecular Medicine, 2015 Mar 13, Volume 20 (Issue 1), Pages 658–666.
[7] Dhiraj Joshi, David Abraham, Xu Shiwen, Daryl Baker, and Janice Tsui (2014) Potential role of erythropoietin receptors and ligands in attenuating apoptosis and inflammation in critical limb ischemia – Journal of Vascular Surgery, Volume 60, Issue 1, July 2014, Pages 191-201.e2.
[8] Masaaki Watanabe, Yu Saito, Jesper Wallmo, Tohru Takahashi, et al (2016) An Engineered Innate Repair Receptor Agonist, ARA 290, Protects Rat Islets from Cytokine-induced Apoptosis – Journal of Diabetes & Metabolism, January 2016, Volume 7 (Issue 10).
[9] Albert Dahan, Michael Brines, Marieke Niesters, Anthony Cerami, and Monique van Velzen (2016) Targeting the innate repair receptor to treat neuropathy – Pain Reports, 2016 Aug 9, Volume 1 (Issue 1), Page e566.
[10] Bo Peng, Gangcheng Kong, Cheng Yang and Yingzi Ming (2020) Erythropoietin and its derivatives: from tissue protection to immune regulation – Cell Death & Disease volume 11, Article number: 79 (2020)
Why Choose Direct Peptides ?
Buy ARA-290 today from Direct Peptides, your trusted source for high-quality peptides. We offer 2mg vials, 15ml and 30ml nasal sprays, and 2 mg pre-mixed pens or cartridges to suit your research needs. Count on Direct Peptides for reliable, high-purity ARA-290 to support your studies.
ALL CONTENT AND PRODUCT INFORMATION AVAILABLE ON THIS WEBSITE IS FOR EDUCATIONAL PURPOSES ONLY.
DISCLAIMER: These products are intended solely as a research chemical only. This classification allows for their use only for research development and laboratory studies. The information available on our Direct Peptides website: https://m.direct-peptides.com is provided for educational purposes only. These products are not for human or animal use or consumption in any manner. Handling of these products should be limited to suitably qualified professionals. They are not to be classified as a drug, food, cosmetic, or medicinal product and must not be mislabelled or used as such.
Related Posts

ARA-290 Neuropathy: A Research Peptide For Rheumatoid Arthritis
This blog investigates the potential use of ARA-290 in treating rheumatoid arthritis (RA), a chronic autoimmune condition characterised by joint inflammation, pain, and long-term damage. It explores the peptide’s anti-inflammatory and neuroprotective properties, highlighting how it may reduce inflammation, protect nerve fibers, and support tissue repair.scription here
ARA-290 In Autoimmune Diseases: Research And Potential
This blog explores how ARA-290 targets the innate repair receptor to reduce inflammation and promote healing without affecting red blood cell production. Studies in conditions like multiple sclerosis and diabetic retinopathy highlight its potential to reduce inflammation and aid repair. Ongoing research is focused on confirming its safety and long-term effectiveness, offering hope for better autoimmune disorder treatments.


